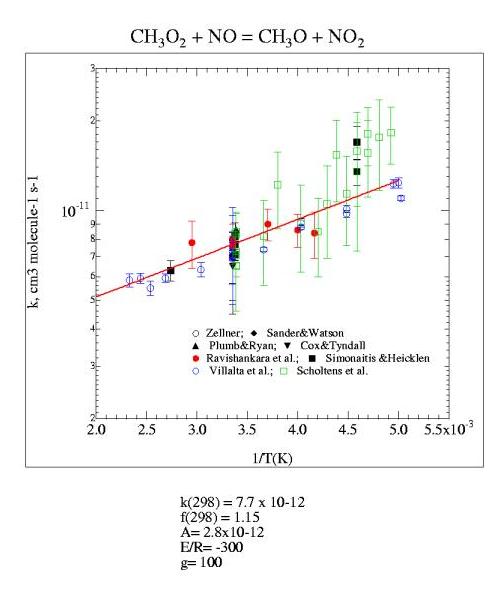
Figure 5. Reaction 3 CH3O2 + NO
Download high resolution image: pdf
Table 5. Reaction 3 CH3O2 + NO
| Reference | Method | Species detected /methoda | k(298 K) b | E/R (K) | Temp range (K) |
|---|---|---|---|---|---|
| Sander and Watson (1980) | FP-UVA | CH3O2/UVA | 7.1±1.4 | 298 | |
| Ravishankara et al. (1981) | LP – LIF | NO2/LIF | 8.1±1.6 | -86±112 | 240-339 |
| Cox and Tyndall (1980) | MM | CH3O2/UVA | 6.5±2.0 | 298 | |
| Plumb et al. (1981) | FT-MS | CH3O2/MS | 8.6±2.0 | 298 | |
| Simonaitis and Heicklen (1981) | FP-UVA | CH3O2/UVA | 7.7±0.9 | -380±250 | 218-365 |
| Zellner et al. (1986) | LP-UVA | CH3O/LIF | 7±2 | 298 | |
| Masaki et al. (1994) | FT-PIMS | CH3O2/PIMS | 11.2±1.4 | 298 | |
| Villalta et al. (1995) | FT-CIMS | CH3O2/CIMS | 7.5±1.3 | -285±60 | 199-429 |
| Scholtens et al. (1999) | TFR-CIMS | CH3O2/CIMS | 7.8±2.2 | -600±140 | 203-295 |
| Sehested et al. (1993) | PR-UVA | NO2/UVA | 8.8±1.4 | 298 | |
| Recommended | 7.7±0.8 | -300±100 |
a) FP: Broad-band flash photolysis, UVA: UV absorption, LP: Laser photolysis, LIF: Laser induced fluorescence, FT: Flow tube, MS: Mass spectrometry, PIMS: Photoionization mass spectrometry, TFR: Turbulent flow reactor, PR: Pulse radiolysis, MM: Molecular modulationb. In units of 10-12 cm3 molecule-1s-1